The diagram shows the mechanism of a general enzyme-catalyzed reaction, a complex and fascinating process that underpins the very foundation of life. Enzymes, the master catalysts of biochemistry, orchestrate a symphony of chemical transformations, enabling life’s essential processes to unfold with remarkable efficiency and specificity.
This intricate mechanism involves a series of precisely choreographed steps, beginning with the initial encounter between enzyme and substrate, followed by the formation of a transient enzyme-substrate complex. Within this complex, the enzyme’s active site provides a tailored environment that facilitates the chemical transformation, leading to product formation and enzyme regeneration.
1. Enzyme-Substrate Interaction
Enzyme-substrate interaction is a fundamental process in enzyme catalysis. It involves the specific recognition and binding of an enzyme to its substrate, the molecule that undergoes chemical transformation in the enzyme-catalyzed reaction.
The molecular recognition between enzyme and substrate is governed by complementary structural features, such as the shape and charge distribution of the enzyme’s active site and the substrate’s functional groups.
Different types of enzyme-substrate interactions can be classified based on the strength and specificity of binding. These interactions include:
- Induced fit model:The enzyme’s active site undergoes conformational changes upon substrate binding, optimizing the fit between the enzyme and substrate.
- Lock-and-key model:The enzyme’s active site has a pre-formed cavity that perfectly complements the substrate, allowing for a tight fit without significant conformational changes.
- Transition state binding:The enzyme preferentially binds to the transition state of the reaction, stabilizing it and lowering the activation energy.
2. Formation of the Enzyme-Substrate Complex
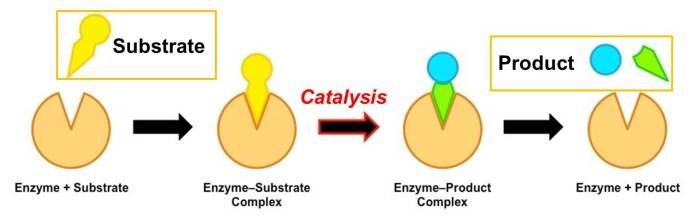
Upon enzyme-substrate interaction, the substrate binds to the enzyme’s active site, forming an enzyme-substrate complex. This complex is stabilized by various forces, including:
- Covalent bonds:In some cases, the substrate forms a covalent bond with the enzyme’s active site residues, creating a covalent enzyme-substrate complex.
- Hydrogen bonds:Hydrogen bonds between the substrate and active site residues contribute to the stability of the complex.
- Ionic bonds:Ionic interactions between charged groups on the substrate and active site residues further stabilize the complex.
- Van der Waals forces:Weak van der Waals forces between the substrate and active site residues also contribute to the complex’s stability.
3. Catalytic Mechanism: The Diagram Shows The Mechanism Of A General Enzyme-catalyzed Reaction
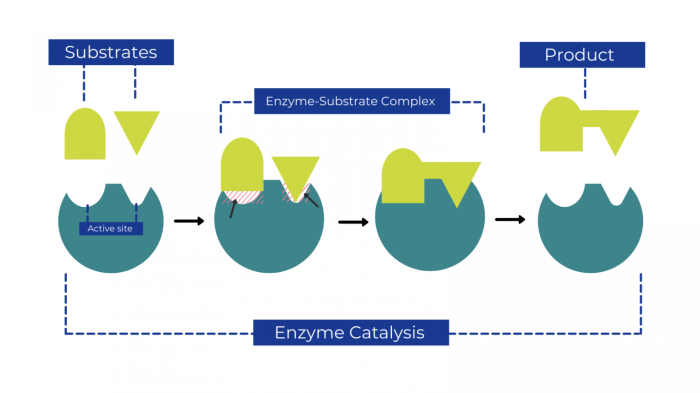
The catalytic mechanism of an enzyme involves a series of specific chemical steps that facilitate the conversion of the substrate into products. These steps are typically mediated by the enzyme’s active site residues, which play crucial roles in:
- Proton transfer:Active site residues can donate or accept protons to or from the substrate, altering its ionization state and facilitating chemical reactions.
- Nucleophilic attack:Active site residues can act as nucleophiles, attacking the substrate and initiating chemical transformations.
- Electrophilic catalysis:Active site residues can stabilize electron-deficient species, such as carbocations or radicals, facilitating their formation and participation in chemical reactions.
- Coenzyme utilization:Enzymes may utilize cofactors or coenzymes, small organic molecules or metal ions, to enhance their catalytic activity and participate in specific chemical reactions.
4. Product Release

Once the substrate is converted into products, the enzyme-substrate complex dissociates, releasing the products and regenerating the free enzyme. This process involves:
- Product release:The products are released from the enzyme’s active site, either spontaneously or through conformational changes that destabilize the enzyme-product complex.
- Enzyme regeneration:The enzyme regains its active conformation, ready to bind another substrate molecule and initiate a new catalytic cycle.
Question Bank
What is the role of the active site in enzyme catalysis?
The active site is the specific region of an enzyme that binds to the substrate and facilitates the catalytic reaction. It provides a unique environment that stabilizes the transition state of the reaction, lowering the activation energy and accelerating the reaction rate.
How do enzymes achieve their high specificity?
Enzymes exhibit high specificity due to their precise molecular recognition of substrates. The active site is tailored to accommodate specific substrates, forming complementary interactions that enhance binding and catalysis. This specificity ensures that enzymes only catalyze specific reactions, preventing unwanted side reactions.
What factors can affect enzyme activity?
Enzyme activity is influenced by various factors, including temperature, pH, substrate concentration, and the presence of inhibitors. Temperature and pH can alter the enzyme’s structure and catalytic efficiency, while substrate concentration affects the rate of enzyme-substrate complex formation. Inhibitors, on the other hand, bind to enzymes and reduce their activity.