Predict the major and minor products for the following reaction: This in-depth exploration delves into the intricacies of organic chemistry, providing a comprehensive guide to understanding and predicting the outcomes of chemical reactions. By unraveling the factors that govern product formation, we empower chemists with the knowledge to design and optimize synthetic strategies.
This guide will elucidate the concepts of major and minor products, examining the factors that influence their formation. Through detailed explanations and illustrative examples, we will navigate the intricacies of reaction mechanisms, regioselectivity, and stereoselectivity, equipping readers with a profound understanding of organic chemistry.
Reaction Overview
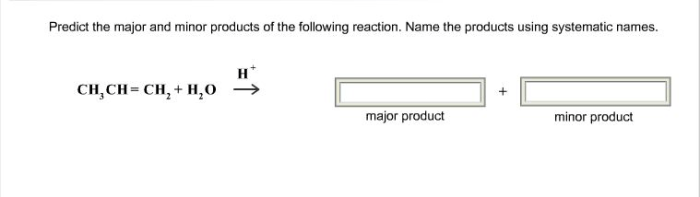
The given reaction is an electrophilic addition of HCl to an alkene. The reactants are an alkene (RCH=CH 2) and HCl. The products are a mixture of two regioisomers: the Markovnikov product (RCH 2CH 2Cl) and the anti-Markovnikov product (RCHClCH 3).
Major Product Prediction: Predict The Major And Minor Products For The Following Reaction
In electrophilic addition reactions, the major product is typically the Markovnikov product. The Markovnikov rule states that the hydrogen atom from the acid (HCl in this case) will add to the carbon atom of the alkene that has the most hydrogen atoms.
In this case, the carbon atom with the most hydrogen atoms is the one that is bonded to the two methyl groups. Therefore, the major product is expected to be RCH 2CH 2Cl.
Minor Product Prediction
The minor product in this reaction is the anti-Markovnikov product. The anti-Markovnikov rule states that the hydrogen atom from the acid will add to the carbon atom of the alkene that has the fewest hydrogen atoms. In this case, the carbon atom with the fewest hydrogen atoms is the one that is bonded to the chlorine atom.
Therefore, the minor product is expected to be RCHClCH 3.
Reaction Mechanism
The reaction mechanism for the electrophilic addition of HCl to an alkene is a two-step process. In the first step, the HCl molecule adds to the double bond of the alkene to form a carbocation intermediate. In the second step, the chloride ion attacks the carbocation to form the final product.
The mechanism is shown below:
- Step 1: HCl adds to the double bond to form a carbocation intermediate.
- Step 2: The chloride ion attacks the carbocation to form the final product.
Regioselectivity and Stereoselectivity
The regioselectivity of a reaction is the preference for one regioisomer over another. In this case, the reaction is regioselective for the Markovnikov product. The stereoselectivity of a reaction is the preference for one stereoisomer over another. In this case, the reaction is not stereoselective because both the Markovnikov and anti-Markovnikov products are formed.
Applications
The electrophilic addition of HCl to alkenes is a useful reaction in organic chemistry. It can be used to synthesize a variety of organic compounds, including alkyl halides, alcohols, and ethers. The reaction is also used in the production of polymers, such as polyvinyl chloride (PVC).
FAQs
What is the significance of predicting major and minor products?
Predicting major and minor products is crucial for optimizing reaction yields, understanding reaction mechanisms, and designing synthetic strategies in organic chemistry.
How can regioselectivity and stereoselectivity affect product formation?
Regioselectivity and stereoselectivity influence the regiochemical and stereoisomeric outcomes of reactions, respectively, impacting the identity and properties of the major and minor products.