Exploring chemical and physical changes lab carolina – Exploring Chemical and Physical Changes: A Comprehensive Guide with Lab Carolina Experiments opens up a fascinating realm of scientific discovery, delving into the intricate differences between chemical and physical transformations. Join us as we embark on an enlightening journey, shedding light on the fundamental principles that govern these intriguing phenomena.
Through engaging experiments and in-depth explanations, this guide will empower you with a profound understanding of chemical and physical changes, equipping you to navigate the complexities of the scientific world with confidence.
1. Introduction
Chemical changes involve the formation of new substances with different chemical compositions, while physical changes do not alter the chemical composition of a substance.
Chemical changes can be recognized by the release or absorption of energy, color changes, or the formation of gas. Examples include burning, rusting, and digestion. Physical changes, on the other hand, involve changes in the form or appearance of a substance without altering its chemical composition.
Examples include melting, freezing, and boiling.
2. Lab Carolina Experiments
Exploring Chemical and Physical Changes Experiment, Exploring chemical and physical changes lab carolina
The Lab Carolina Exploring Chemical and Physical Changes experiment demonstrates the differences between chemical and physical changes through various experiments.
The purpose of the experiment is to:
- Observe and identify chemical and physical changes.
- Understand the characteristics of chemical and physical changes.
- Apply the concepts of chemical and physical changes to real-world scenarios.
Materials needed:
- Iron filings
- Sulfur powder
- Water
- Graduated cylinder
- Beaker
- Stirring rod
- Test tubes
- Safety goggles
- Lab coat
3. Procedures
Safety Precautions:
- Wear safety goggles and a lab coat.
- Do not mix chemicals unless instructed.
- Dispose of chemicals properly.
Step-by-Step Instructions:
- Physical Change:Place 10 mL of water in a beaker. Add a few drops of food coloring. Stir the solution to observe a physical change.
- Chemical Change:In a test tube, mix a small amount of iron filings and sulfur powder. Heat the test tube gently over a flame. Observe the color change and the release of gas.
- Physical Change:Melt a small piece of wax in a test tube. Allow the wax to cool and solidify. Observe the physical change.
- Chemical Change:Add a few drops of vinegar to a small piece of baking soda in a test tube. Observe the formation of gas and the release of energy.
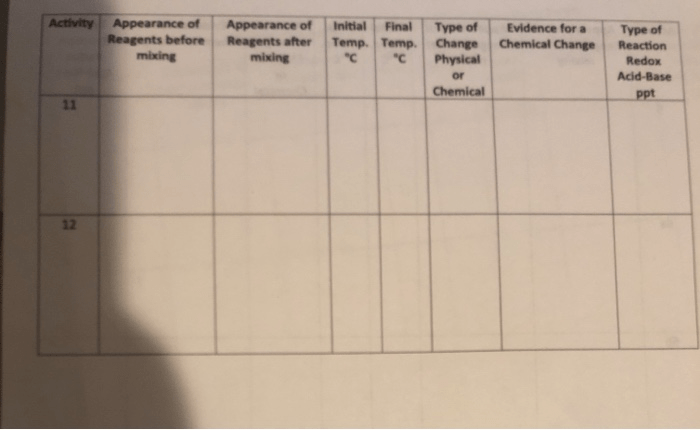
Data Collection and Recording:
Record your observations in a table, including the type of change (chemical or physical), the substances involved, and the characteristics of the change.
4. Results: Exploring Chemical And Physical Changes Lab Carolina
Expected Results:
- Physical Change:The water will change color when food coloring is added.
- Chemical Change:The iron filings and sulfur powder will react to form a black solid and release a gas.
- Physical Change:The wax will melt when heated and solidify when cooled.
- Chemical Change:The baking soda and vinegar will react to form carbon dioxide gas and release energy.
Data Interpretation:
The table should show the type of change, the substances involved, and the characteristics of the change for each experiment.
Key Questions Answered
What is the difference between a chemical change and a physical change?
A chemical change involves the rearrangement of atoms to form new substances, while a physical change alters the form or appearance of a substance without changing its chemical composition.
What are some examples of chemical changes?
Burning, rusting, and cooking are all examples of chemical changes.
What are some examples of physical changes?
Melting, freezing, and boiling are all examples of physical changes.